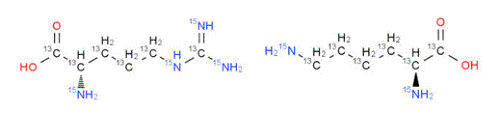
Isotopically Labeled Peptides
21st Century Biochemicals is one of the world’s leading manufacturers of Isotopically
labeled peptides. Using highly enriched 13
C
/15
N
or both 13
C
/15
N
, and 2
D
labeled amino
acids (99+% isotopic enrichment), researchers are utilizing our peptides for many
different proteomic applications.
• 100% Manufactured in Marlborough, MA
• Available in 96 well plate or 2D bar coded Matrix tube format, individual tubes,
freeze dried or liquid form; purity to >97% and a variety of scales to fit your
research needs!
• The most rigorous QC in the world – all high purity peptides include tandem MS to
confirm each high purity peptide in addition to HPLC and ESI-MS analysis (in-
house AAA also available)
• >99% isotopic purity
• Peptides can be supplied as TFA acetate or HCL salt forms
Our peptide QC is the most rigorous in the industry and includes ESI-MS and HPLC
analysis as well as tandem MS to confirm each peptide sequence. Amino acid analysis
(performed in-house) can also be added to provide net peptide content. We manufacture
over 4,000 purified isotopically labeled and over 20,000 high-throughput peptides (96-well
format) each year and growing!
There are many options to consider when ordering isotopically labeled peptides. Some
of the options include:
Isotopic Purity – 99+% (for 13
C
/15
N
-labeled amino acids)
Amount of material required – 1nmol, 10nmol, 100nmol, 1mg, 5mg, up to gram quantities
Peptide purity - desalted, >70%, >80%, >95%, >95%. >97%; >98% available for certain peptides,
please inquire
Labeled amino acids used: nearly all amino acids are available 13
C
/15
N
-labeled and properly pro-
tected for use in custom peptide synthesis
C-terminal labeled: tryptic digestion utilizes peptides with C-terminal 13
C
/15
N
-Arg or Lys; 13
C
/15
N
-
Phe or Tyr used for chymotrypsin digestions; other amino acids available
Internal labeled: most amino acids are available; pricing varies dependent upon the specific
amino acid and scale requested and the purity required
Packaged as: Peptide mixtures, lyophilized or liquid aliquots, labeled peptide arrays, balanced la-
beled/unlabeled peptide pairs, many other options available!
Citations
Our scientists work with some of the most distinguished laboratories and proteomic centers across
the globe. Our CSO was also part of the CPTAC (Clinical Proteomic Tumor Analysis Consortium)
Working Group that recently published recommendations for the production of optimal peptides for
MS-based (e.g., SRM/MRM) assays:
Recommendations for the Generation, Quantification, Storage, and Handling of Peptides
Used for Mass Spectrometry–Based Assays. Andrew N. Hoofnagle, Jeffrey R. Whiteaker, Steven
A. Carr, et al. Clin. Chem., Jan 2016; 62: 48 - 69.
CPTAC packages are also available which include epitope design, antibody production, along with
isotopically-labeled peptides for use in biomarker discovery platforms.
We are also proud to the supplier to some of the world’s foremost scientists and scientific institu-
tions, including the: Broad Institute of MIT/Harvard, Harvard Medical School, Pfizer, NIH, KIST
(Korean Institute for Science and Technology), Fred Hutchinson Cancer Center, FDA, University of
Washington, UT-Southwestern Medical Center, Dana Farber Cancer Institute, and many, many oth-
ers (see examples of publications below).
The Broad Institute of MIT and Harvard:
Simplified and Efficient Quantification of Low-abundance Proteins at Very High Multiplex via
Targeted Mass Spectrometry. Michael W. Burgess, Hasmik Keshishian, D. R. Mani, Michael A.
Gillette, and Steven A. Carr. Mol. Cell. Proteomics, Apr 2014; 13: 1137 - 1149.
The Korean Institute for Science and Technology and Seoul National University:
Both Targeted Mass Spectrometry and Flow Sorting Analysis Methods Detected the Decreased
Serum Apolipoprotein E Level in Alzheimer's Disease Patients. Sun-Ho Han, Jun Seok Kim,
Youngju Lee, Heesun Choi, Jong-Won Kim, Duk Lyul Na, Eun Gyeong Yang, Myeong-Hee Yu,
Daehee Hwang, Cheolju Lee, and Inhee Mook-Jung. Mol. Cell. Proteomics, Feb 2014; 13: 407
- 419.
The FDA, College Park, MD:
Quantification of Allergenic Bovine Milk αS1-Casein in Baked Goods. Using an Intact 15N-Labeled
Protein Internal Standard. G. Asher Newsome and Peter F. Scholl* J. Agric. Food Chem. 2013, 61,
5659−5668.
Fred Hutchinson Cancer Center/University of Washington:
Sequential Multiplexed Analyte Quantification Using Peptide Immunoaffinity Enrichment Coupled to
Mass Spectrometry. Jeffrey R. Whiteaker, Lei Zhao, Chenwei Lin, Ping Yan, Pei Wang, and Amanda
G. Paulovich. Mol. Cell. Proteomics, Jun 2012; 11: M111.015347.
Pfizer:
Identification and quantification of osteopontin splice variants in the plasma of lung cancer patients
using immunoaffinity capture and targeted mass spectrometry. Jiang Wu, Pooja Pungaliya, Eugenia
Kraynov, and Brian Bates. Biomarkers, 2011, 1–9, 20 November 2011
The Broad Institute of MIT and Harvard and the Fred Hutchinson Cancer Center:
Evaluation of Large Scale Quantitative Proteomic Assay Development Using Peptide Affinity-based
Mass Spectrometry. Jeffrey R. Whiteaker, Lei Zhao, Susan E. Abbatiello, Michael Burgess, Eric
Kuhn, Chen Wei Lin, Matthew E. Pope, Morteza Razavi, N. Leigh Anderson, Terry W. Pearson,
Steven A. Carr, and Amanda G. Paulovich. Mol. Cell. Proteomics, Apr 2011; 10: M110.00564.
Overview
Custom Peptide Synthesis
Isotopically Labeled Peptides
Solubilizing Labeled Peptides
What Purity is Right for My
Peptide?
Peptide Synthesis

100% MADE IN THE USA Since 2003 ISO 9001:2015 Certified Peptide Manufacturer